Dr. Lew Feldman, Garden Director
Water Transport
How do plants “drink” water?
In this issue of IGYA we consider the process of water movement in plants, over great distances, such as to the tops of tall trees, without the aid of a pump. To appreciate how plants carryout this great feat, we need to consider three elements:
- Some properties of the water molecule,
- The fact the water evaporates, moving from a liquid to a gaseous phase and,
- The pathway (plumbing system) along which water moves
1. “Sticky” Connections
The first characteristic to consider is that, in its liquid state each water molecule is joined (attached) to other water molecules; they are thus said to be “sticky”.
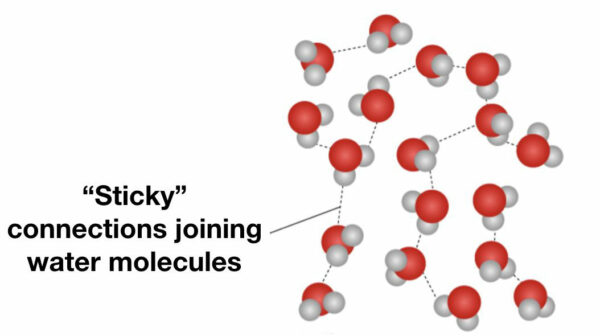
(Modified from Beverly High School drawing)
This stickiness can easily be observed by taking a paperclip and gently placing it on the surface of a glass of water. Even though the paperclip is denser (heavier) than the water it does not sink. This is because the water molecules are attached to each other (“sticky”), forming a net-like structure that supports the paperclip and prevents it from sinking.

A paperclip floating on the water surface illustrates the characteristic of one water molecule attaching to another (“stickiness”).
2. Water Evaporation
The second feature we need to consider is that water evaporates, going from a liquid to a gaseous state. When evaporation occurs the stickiness (the connection of one water molecule to another) is broken.
3. The Plumbing System
The third characteristic that underlies water movement in plants is that the majority of water movement occurs in dead cells, which are joined end-to-end, forming an empty (hollow, open) tube-like pathway in which water exists as a continuous, unbroken column.
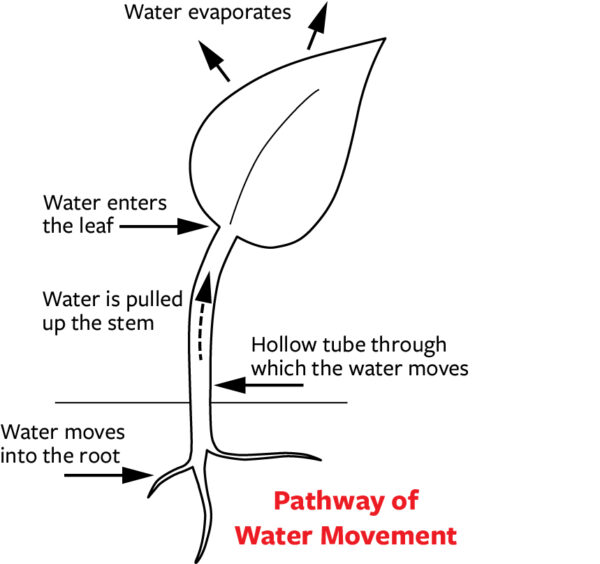
Having been introduced to the three elements underlying water movement, let’s consider how water moves from the roots to the leaves. To understand this process we need to appreciate that the driving force (the energy) for water movement comes from the sun. As the sun heats the atmosphere, water molecules evaporate from the leaves, and in doing so break the sticky bonds which connected one water molecule to another, similar to what happens when you hang clothes on a line to dry. The breaking of the sticky bonds occurs when the force to evaporate becomes stronger than the strength of the stickiness connecting two water molecules.
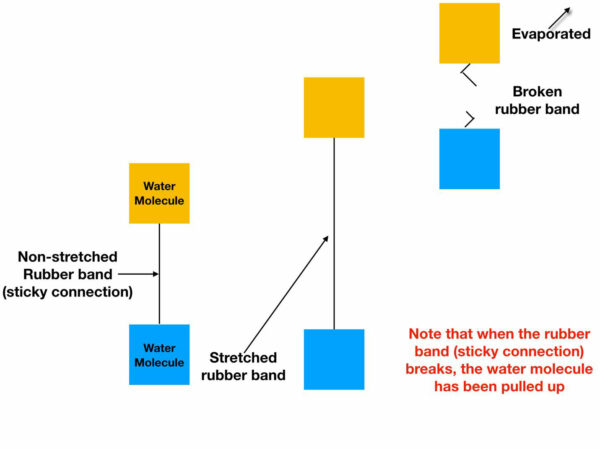
A way to visualize what is happening during evaporation is to view the sticky connection between two water molecules (colored yellow and blue, below) as equivalent to a rubber band. As the rubber band is stretched, a tension, or a pull is created between water molecules. And since all the water molecules in the plumbing system are connected, this tension, or pull exists in the entire column of water. As the stretching of a rubber band increases, at some point the rubber band will be stretched so much that it breaks. This breaking of the rubber band can be viewed as equivalent to the breaking of stickiness between water molecules as they evaporate.
Evaporating Water Molecules
And here is the “secret” of water movement; as a water molecule evaporates, the accompanying stretching of the sticky connections pulls up another water molecule to replace the one which has just evaporated; equivalent to the blue water molecule moving into the position formerly occupied by the yellow water molecule, which has just evaporated (as pictured below). In this way water moves to the tops of tall trees; water is essentially pulled up by the evaporating molecules.